Class 12 Physics Chapter 1 – Electric Charges and Fields
Forces between Two Point Charges – Coulomb’s Law, Coulomb’s Law in Vector Form
[Watch Video to Understand in Detail and in Malayalam Language]
Coulomb’s Law
Coulomb’s law states that the force between two point charges varied inversely as the square of the distance between the charges and was directly proportional to the product of the magnitude of the two charges and acted along the line joining the two charges.
Thus, if two point charges q1, q2 are separated by a distance r in vacuum, the magnitude of the force (F) is
and
In free space or vacuum the value of constant k
is called the permittivity of free space. The value of
in SI units is
So that Coulomb’s law is written as
The Coulomb’s law and the value of gives us an idea about SI unit of charge coulomb. Let q1 = q2 = 1 C and r = 1 m, then force
That is, 1 C is the charge that when placed at a distance of 1 m from another charge of the same magnitude in vacuum experiences an electric force of repulsion of magnitude 9 X 109 N. 1 C is too big unit to be used. In practice, in electrostatics, one uses smaller units like 1 mC or 1 µC.
Coulomb’s law in Vector Notation
Since force is a vector, we have to write Coulomb’s law in the vector notation [Note: here we are using bold letters to represent vectors]. Let the position vectors of charges q1 and q2 be r1 and r2 respectively. The resultant vector leading from q1 to q2 is denoted by
r21 = r2 – r1
And the vector leading from q2 to q1 is denoted by r12:
r12 = r1 – r2 = – r21
The unit vectors along this can be represented as:
Also force on q1 due to q2 by F12 and force on q2 due to q1 by F21.
Coulomb’s force law between two point charges q1 and q2 located at r1 and r2 in vacuum or free space is then expressed as
See figure for geometric representation.
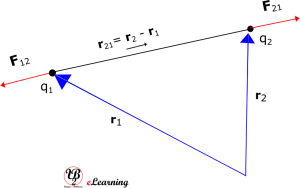
The above diagram is for q1 x q2 > 0, the force is repulsive in nature. If q1 x q2 < 0, the force is attractive in nature and figure below shows that.
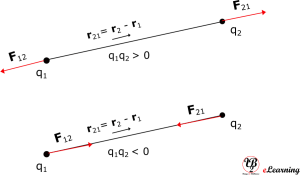
Some points related to Coulomb’s law in vector notation are as follows:
It is valid for any sign of q1 and q2 whether positive or negative. If q1 and q2 are of the same sign (either both positive or both negative), F21 is along , which denotes repulsion, as it should be for like charges. If q1 and q2 are of opposite signs, F21 is along
, which denotes attraction, as expected for unlike charges. That is equation is true for both cases correctly.
It also proves that Coulomb’s law agrees with Newton’s third law.
The force F12 on charge q1 due to charge q2, is obtained by simply interchanging 1 and 2 in the above equation, i.e.,
